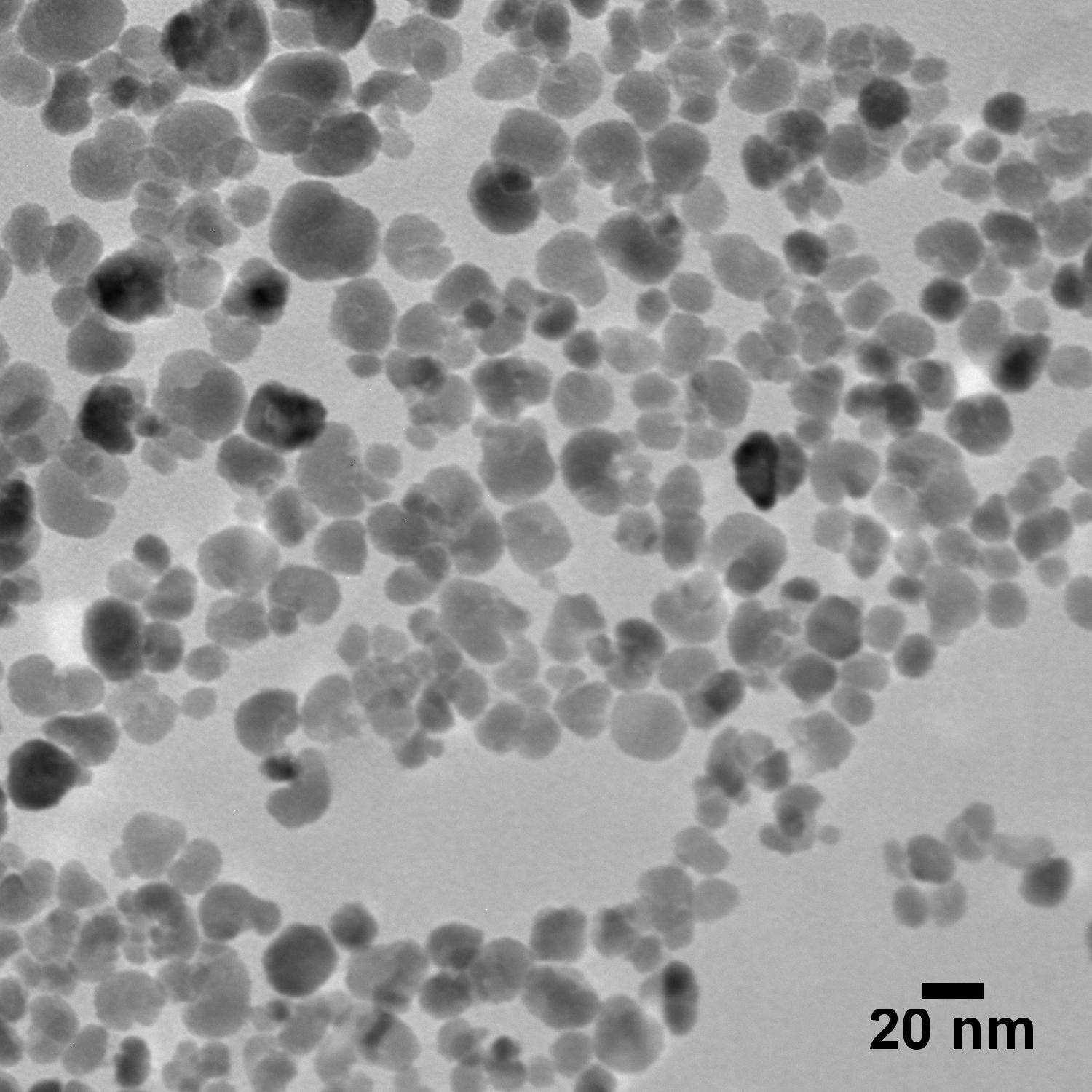
Nanomaterials for Targeted Drug Delivery & Photothermal Therapy
At Fortis Life Sciences, we utilize our proprietary nanoparticle manufacturing techniques to provide solutions for the rapid development and commercialization of medical devices and therapeutic products. There are many nanomaterial companies and many GMP manufacturers but very few that specialize in both.
With our diverse expertise in nanoparticle fabrication and biofunctionalization for targeted delivery, controlled-release, photothermal therapy, and more, we help partners bring high-impact nano-enabled therapeutic products to market.
Our CDMO services include:
- Research: Proof-of-Concept Synthesis & Characterization
- Development: Optimization, Scale-Up, Process Development & Validation
- Production: Transfer to & Scaled Manufacturing
Key Platforms
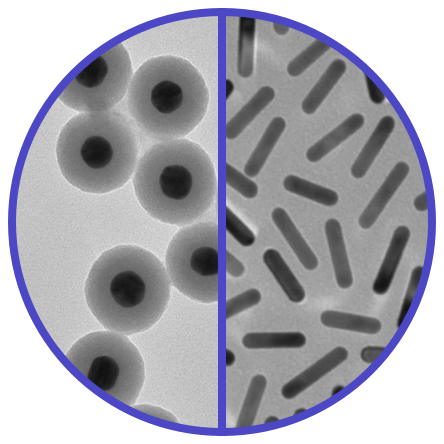
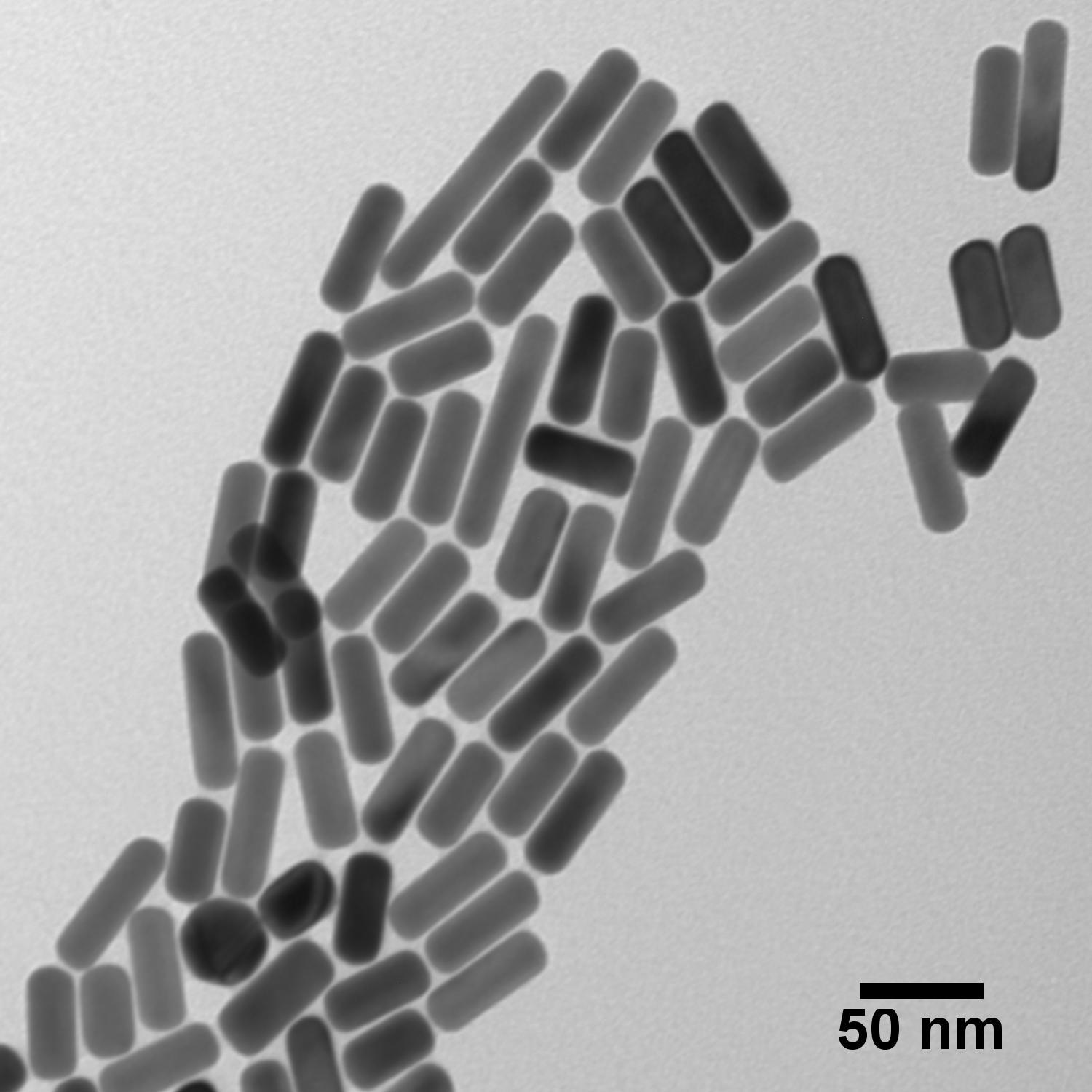
Metal Nanoparticles
- Gold, Silver, More
- Spheres, Rods, More
- Silica-Shelled Metal Core
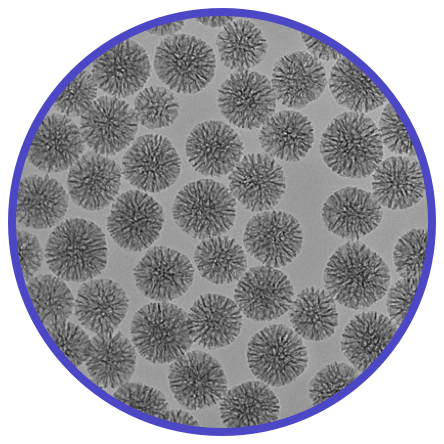
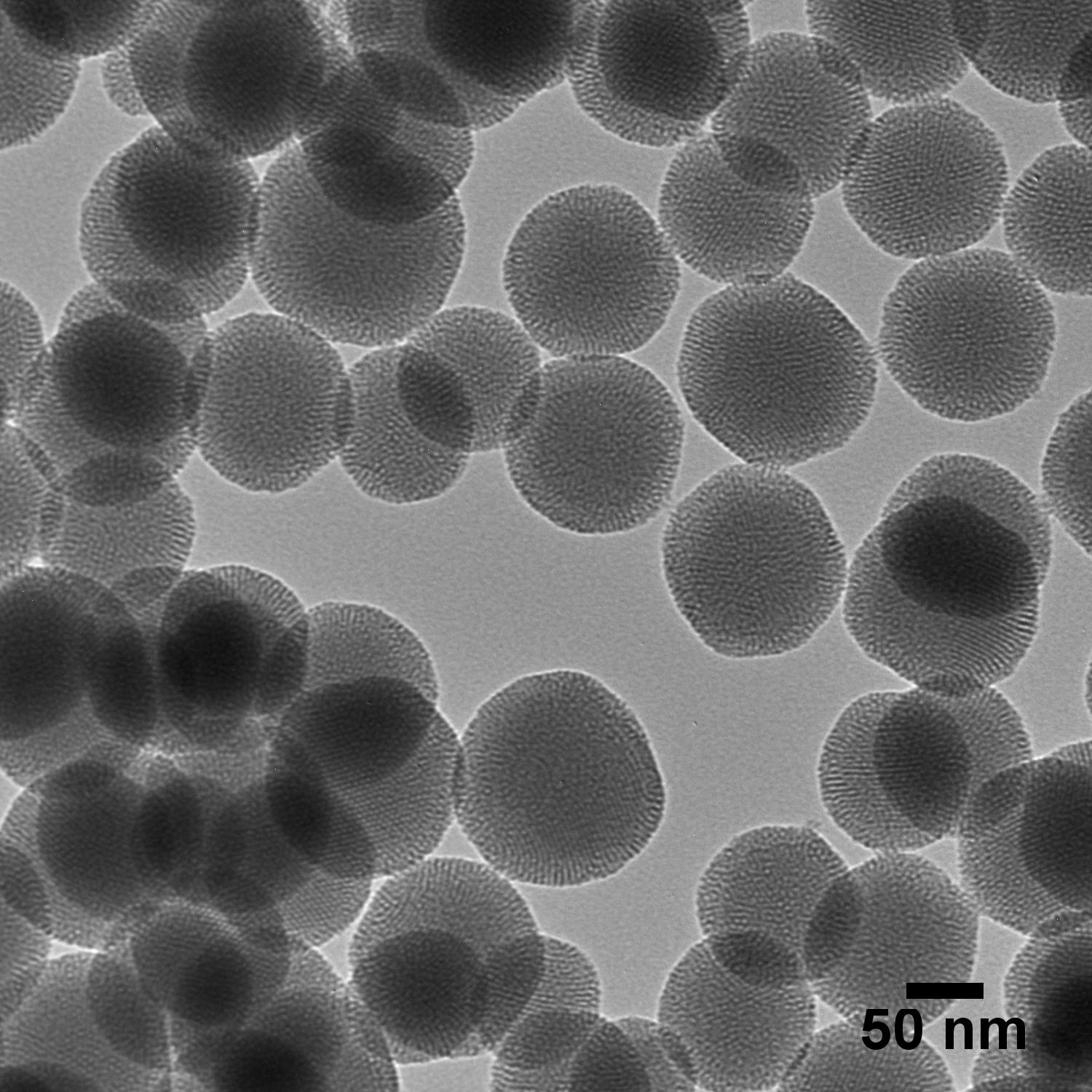
Silica Nanoparticles
- Mesoporous
- Microporous/Solid
Polymer Nanoparticles
- PLGA, PLA
- Lipid
- More
Application Areas
Targeted Delivery
Owing to their ability to rapidly and accurate deliver a therapeutic agent, gold, silica, magnetite, and polymer (PLGA) nanoparticles all have demonstrated efficacy in targeted drug delivery applications.1
Theranostics
Nanoparticles solve the unique combinations of challenges presented by the emerging area of Theranostics. With their ability to rapidly and accurately accumulate on a target of interest, they can simultaneously gather biochemical and morphological information about the target while efficiently delivery a therapeutic compound.2
Photothermal Therapeutics
Metallic nanoparticles like gold nanoshells, gold nanorods, and magnetite nanoparticles have unique plasmonic properties that make them highly effective in photothermal therapeutic applications ranging from topical treatments to cancer therapies.3,4
Gene Delivery
Recently, there has been a rapid growth in exploration of mesoporous silica nanoparticles as platforms for gene delivery. With their tunable particles size, pore size, and pore geometry, mesorporous silica nanoparticles are considered an ideal platform for the targeted delivery of therapeutic genes.5,6
Case Studies
-
Client: Biotechnology company
Customer approached us for the development of a cell-based cancer therapy utilizing mesoporous silica. Following a feasibility scale synthesis that produced material meeting specifications, development progressed to scale up efforts, moving from mg scale to 50 gram batches. On-going work supports further scale up efforts with the implementation of design controls to move production under our QMS system for GMP manufacturing.
Development and On-Going Manufacturing Work Includes:
- Controlled design history file and all documentation under design controls.
- Delivery of quality plan and analytics with Process Failure Mode and Effects Analysis (pFMEA) to identify the risks in the material manufacturing
- Bill of Materials (BOM-SPEC) with qualified suppliers
- Developing a Manufacturing Master Record (MMR), including Master Batch Records (MBR)
- Certificates of Analysis (CoAs) or Certificates of Conformance (CoC)
- Creation of process documentation including material specifications, SOPs, and work instructions.
- Transfer of scaled manufacturing process to phase appropriate (compliant to FDA Phase 1 guidance) environment, including implementation of sterilization processes and sterility testing.
- Validation runs repeatedly producing the material under all the necessary controls to ensure the material meet all criteria of the formal Product Specification
-
Client: Biotechnology company
Customer required a partner to develop a biofunctionalized gold nanoparticle for targeted delivery of a therapeutic oligo. The particle needed to be size appropriate for the application with a low coefficient of variation, offer high biocompatibility, and provide targeted delivery of the therapeutic molecule. Work at our end began by adapting a laboratory scale procedure provided by the customer and progressed to sweeping conjugation and loading conditions to determine the optimal procedure. Future work will select the best mode synthesis from the prior phase based on characterization data and functional testing and begin outlining the development effort to scale and GMP manufacture.
Development Work Included:
- Transfer of provided procedure to our facility
- Full characterization of synthesized particles, including:
- Hydrodynamic Diameter by DLS
- Stability over time by DLS and UV-Vis
- Zetapotential by DLS
- Oligo Loading by custom assay
- Functional verification of oligo binding by Lateral Flow Immunoassay
- Delivery of a 1 mL sample of each conjugate variant
-
Client: Sienna Biopharmaceuticals
Sienna Biopharmaceutical partnered with us to co-develop a photothermal acne treatment made of nanoparticles. The technology is made possible by highly concentrated silica coated silver nanoplates that are introduced into the hair follicles of over-productive oil glands. Standard dermatology lasers are then used to locally heat and impair the nearby sebaceous glands, reducing oil production and acne. In order to make this therapy successful, the silver nanoplates needed to be protected from the skin’s local environment. We worked closely with Sienna to design and optimize the silica encapsulation, maximizing the formulation’s stability on skin and thereby increasing treatment efficacy.
Transfer to GMP manufacturing under QMS: We developed a working prototype and helped Sienna define a formal Product Specification, a critical early phase of GMP development. The transfer to GMP manufacturing for clinical supply and preparation for commercial production included:
- Repeatedly producing the material under all the necessary controls to ensure the material meet all criteria of the formal Product Specification
- Establishing a process flow diagram based on the R&D process and control points for In Process Quality Control (IPQC)
- Developing a Manufacturing Master Record (MMR), including Master Batch Records (MBR) and a Bill of Materials with qualified suppliers
- Process development to increase the standard batch size 100×
- Process Failure Mode and Effects Analysis (pFMEA) to identify the risks in the material manufacturing
- Several iterations of robustness work to ensure a consistent manufacturing process at commercial scale
- Successful audits to ensure QSR & ISO13485 requirements were met
-
Client: Private medical device company dedicated to dermatology therapies
This customer had been producing a proprietary topical therapeutic with a nanoparticle component and required a second supplier for their commercial technology. We worked with them to replicate their established synthesis protocols, testing, and release procedures under our QMS so that we could be qualified as a second supplier for the nanoparticle component.
GMP process development included:
- Client provided us with their existing Manufacturing Master Record (MMR) and Batch Records and the synthesis was repeated in our R&D laboratory
- After developing a Quality Plan for the project, we transferred and adapted the Batch Records and related into our QMS and performed engineering runs to qualify the process
- Additional test methods were developed and validated and new suppliers were qualified
- We produced a cGMP compliant qualification batch and delivered the material to the client for evaluation
- At the successful completion of the project, the client approved us as a verified second source
- We were reviewed by the Client’s Notified Body and added as a Crucial Supplier to the Client’s CE Certification
- We became the primary supplier of the material to the Client
Related Products for Research Use
Talk to Our Development Team
References
- Mitchell, M.J., Billingsley, M.M., Haley, R.M. et al. Engineering precision nanoparticles for drug delivery. Nat Rev Drug Discov 20, 101–124 (2021). https://doi.org/10.1038/s41573-020-0090-8
- Xie J, Lee S, Chen X. Nanoparticle-based theranostic agents. Adv Drug Deliv Rev. 2010;62(11):1064-1079. doi:10.1016/j.addr.2010.07.009
- Chatterjee, D. K.; Diagaradjane, P.; Krishnan, S. "Nanoparticle-mediated Hyperthermia in Cancer Therapy" Ther. Deliv. 2011, 2 (8), 1001-1014.
- Jacque, D.; Martínez Maestro, L.; del Rosal, B.; Haro-Gonzalez, P,; Benayas, A.; Plaza, J. L.; Martín Rodríguez, E.; García Solé, J. "Nanoparticles for Photothermal Therapies" Nanoscale 2014, 6, 9494-9530.
- Yixian Zhou, Guilan Quan, Qiaoli Wu, Xiaoxu Zhang, Boyi Niu, Biyuan Wu, Ying Huang, Xin Pan, Chuanbin Wu, Mesoporous silica nanoparticles for drug and gene delivery, Acta Pharmaceutica Sinica B, Volume 8, Issue 2, 2018, Pages 165-177, ISSN 2211-3835, https://doi.org/10.1016/j.apsb.2018.01.007.
- Li, Z.; Barnes, J. C.; Bosoy, A.; Stoddart, J. F.; Zink, J. I. Mesoporous Silica Nanoparticles in Biomedical Applications. Chem. Soc. Rev. 2012, 41 (7), 2590–2605. doi.org/10.1039/C1CS15246G.
By clicking “Acknowledge”, you consent to our website's use of cookies to give you the most relevant experience by remembering your preferences and to analyze our website traffic.